Language


ECMO (Extracorporeal Membrane Oxygenation) equipment is an advanced life support system that temporarily replaces heart and lung functions in critically ill patients. The system consists of a control console that manages operations, a drive unit with pumps to circulate blood, a membrane oxygenator for gas exchange, a water tank for temperature regulation, and an air-oxygen blender to control oxygen concentration. It works by removing blood from the patient's body, oxygenating it and removing carbon dioxide through an artificial membrane lung, then returning the oxygenated blood to the patient's circulation. This technology is crucial for treating severe cardiac or respiratory failure, cardiogenic shock, and supporting patients awaiting organ transplants or recovering from major cardiac surgery.
The control console of ECMO equipment controls the operation of the drive unit and monitors its parameters. After each use, cleaning must be performed according to product requirements: first turn off the power switch on the back of the ROTAFLOW console, then wipe the surface with a 75% ethanol lint-free cloth, followed by wiping with a sterile water-moistened lint-free cloth. When wiping, pay attention to equipment butt
ons and connection interfaces. Never spray liquid on the equipment to prevent liquid from entering the console and causing damage.
The ECMO equipment water tank functions to heat blood temperature to match body temperature, using a heating pump-like method. Heated water is driven by the impeller through the outlet tubing into the membrane lung, exchanges heat with blood, and circulates back to the water tank in an independent circulation loop. The tank must use sterile water for injection and cannot be substituted with saline. The sterile water must be replaced after each patient use to prevent cross-infection.
Cleaning method:
External surfaces can be wiped with 75% ethanol lint-free cloth, followed by sterile water-moistened lint-free cloth wiping
Internal cleaning can be done through bypass mode using the console function to circulate sterile water for injection 3 times
Tubing can be dismantled from the lower front port, soaked in 75% ethanol in a suitable container, then internally flushed with sterile water and externally wiped with lint-free cloth
ECMO equipment drive units mainly include centrifugal pumps and roller pumps, which are the power source of ECMO, carrying out the "circulation" function for blood. After each use, wipe surface coupling agent and other contaminants with 75% ethanol lint-free cloth, followed by sterile water-moistened lint-free cloth. As there are multiple sensors inside the equipment, prevent liquid from entering internally to avoid damage to circuits and sensors. Special note: the drive unit is precise, avoid collision with other equipment or hard objects during cleaning.
The air-oxygen blender is an important device of ECMO equipment, mainly used to control inhaled oxygen concentration and flow rate, reducing patient's inhaled oxygen concentration to a safe range while avoiding side effects of pure oxygen inhalation. When oxygen concentration meets clinical treatment needs (non-traditional pure oxygen inhalation treatment), it is delivered to ECMO. Equipment performance should be tested before use, and if damaged, stop use immediately and change. After use, first clean visible contamination on component surfaces with lukewarm sterile water and cleaner, then wipe surfaces and air-oxygen high-pressure tubes with 75% ethanol lint-free cloth, followed by wiping with sterile water-moistened lint-free cloth.
Based on ECMO equipment characteristics, surface disinfection can use three methods: 75% ethanol, 70% isopropanol, and ultraviolet radiation. See Figure 1 below.
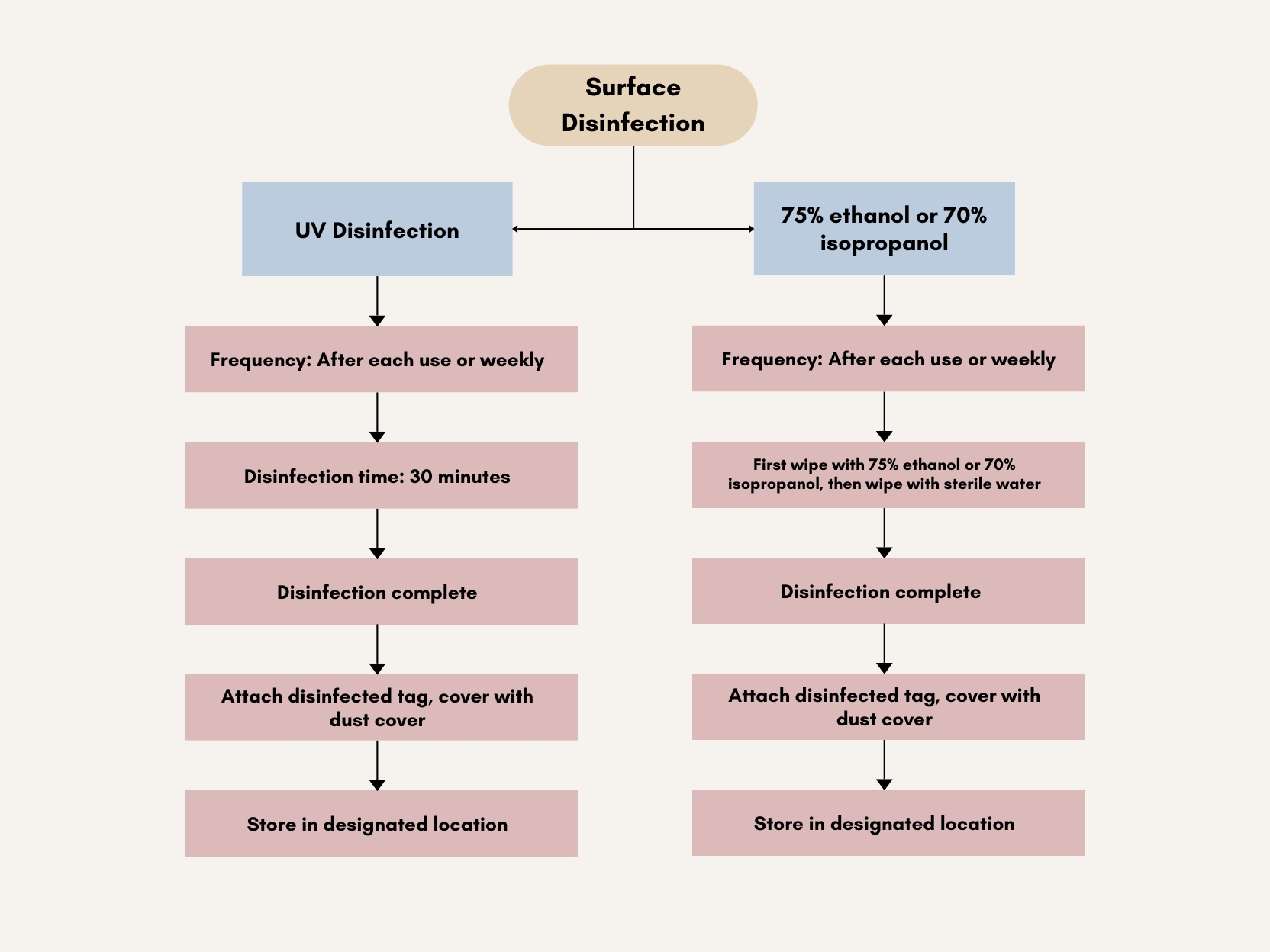
Figure 1. Surface Disinfection Flow Chart for ECMO Equipment
First wipe with 75% ethanol lint-free cloth, then wipe with sterile water-moistened lint-free cloth. Disinfection cycle is after each patient use. Allow to air dry naturally, attach disinfected tag, cover with dust cover, and store in designated location. Keep ethanol away from heat sources to prevent fire.
First wipe with 70% isopropanol lint-free cloth, then wipe with sterile water-moistened lint-free cloth. Disinfection cycle is after each patient use. Allow to air dry naturally, attach disinfected tag, cover with dust cover, and store in designated location.
Ultraviolet wavelength range is 200-275nm, with strongest germicidal effect at 250-270nm, capable of killing various microorganisms including bacterial cells, spores, mycobacteria, viruses, fungi, rickettsia, and mycoplasma. ECMO equipment UV disinfection mainly targets surface disinfection through direct exposure for 30 minutes, then cover with dust cover to reduce contamination risk. Notably, while UV has strong bactericidal effects, it can harm humans. Protect eyes, face, and exposed skin during use, especially eyes being most vulnerable. Never look directly at UV tubes to prevent injury; use UV protective goggles or face shields when necessary.
(1) Visual inspection
Check power cord for exposed or damaged insulation; Change immediately if found to prevent electric shock and fire hazards
Check buttons and knobs for damage
Check drive unit connection interfaces for damage or deformation
Check power indicator lights for normal operation
(2) Function testing Start equipment using tube clamp, clamp tube and press zero button on panel, check zeroing, then set specific speed to 2500r/min, 4L/min, observe fan noise level, verify drive unit operates normally and smoothly.
(3) Flow zero drift test Run equipment at 2500r/min and 4L/min for 20 min, stop until pump stops running, observe if flow display returns to zero or shows -0.05-0.05L/min.
As ECMO equipment generates heat during operation, fans cool through bottom and coupling joint ventilation ports. Clean ventilation ports quarterly to prevent dust accumulation causing poor heat dissipation, equipment alarms, and operation stoppage.
Check:
Button damage, verify temperature changes when pressing up/down keys
After heating, verify temperature changes and matches set value
Confirm pump impeller rotation using equipment circulation loop with bypass tubing
(1) Capacity testing Run equipment continuously until low battery alarm, confirm [LOWBATT] display, verify voltage is 19-20V, then connect AC power, confirm battery low voltage alarm automatically clears, observe battery charging indicator remains solid before fully charged.
(2) Status testing Normally, battery charges fully within 8.5h and runs 89min at full charge. Change battery if charging exceeds 8.5h or full charge runtime is less than 89min. Change battery every 2 years.
As equipment parts contacting patients are non-electrical, risk of membrane lung electric shock is low. Test electrical safety when necessary. Include insulation resistance, protective ground resistance, ground leakage current, and enclosure leakage current per IEC60601-1 standard safety requirements.
ECMO equipment consumables are specialized single-use items with standard infection control requirements:
Check package integrity before use; do not use if packaging incomplete or damaged
Check product model and batch number
Check validity period; prevent contamination during inspection as re-sterilization impossible; regular patient consumables can be disposed in medical waste bags
For infectious disease and high-risk patients, spray with chlorine disinfectant, double-bag in medical waste bags separately, seal properly, clearly mark bags, arrange specialized disposal
Note particularly that used consumables are key infection sources. Exercise careful personal protection when removing consumables to prevent infection from blood or fluid splashes. Must wear gloves, goggles, N95 mask, protective suit, and protective boots.
Wipestar specializes in manufacturing high-quality lint-free wiping cloths specifically designed for professional medical equipment cleaning. Our product line includes both dry and pre-saturated options, engineered to meet the stringent cleanliness requirements of medical devices and critical healthcare environments.
Tags:Medical Equipment
RELATED RESOURCES
Guidelines for Cleaning and Disinfection of Common Medical Equipment
Comprehensive guidelines for medical equipment cleaning and disinfection. Essential protocols for healthcare f......
More
External Cleaning and Disinfection Protocol for Hemodialysis Machines
Comprehensive protocol for external cleaning and disinfection of hemodialysis machines, including step-by-step......
More

How to maintain microfiber cleaning cloth? 6 golden rules to extend its life by 5.8 times
Professional-grade microfiber cleaning cloth maintenance guide: Scientifically extend tool lifeData from revol......
More

Sub-fine Cleanroom Wipes: How Ultrafine Fibers Improve Semiconductor Cleaning Efficiency by 97.6%
Asia Dirtless Cloth: Cleaning Innovation Solutions in the Field of Precision ManufacturingBreakthrough in mate......
More
Related Products
Room 101, Building 1, Angeer Factory, No.4, Hetian Road, Shatian Community, Kengzi Street, Pingshan District, Shenzhen, Guangdong, P.R. China 518122
info@wipestar.com
+86-755-89616775
+86-755-89616773
Related Products
RELATED RESOURCES

Guidelines for Cleaning and Disinfection of Common Medical Equipment
Comprehensive guidelines for medical equipment cleaning and disinfection. Essential protocols for healthcare f.........
More

External Cleaning and Disinfection Protocol for Hemodialysis Machines
Comprehensive protocol for external cleaning and disinfection of hemodialysis machines, including step-by-step.........
More
WIPESTAR
微信官方公众号